Authors: Pamela Opfer and Dan McGrath, OSU Dept. of Horticulture
Cabbage Looper (Trichoplusia ni) & Alfalfa Looper (Autographa californica)


Cabbage looper & Alfalfa looper larvae are very similar Cabbage looper adult moth (Photos by Ken Gray)

Alfalfa looper adult moth (Photos by Ken Gray)
How to ID Pest
It is very difficult to separate the larvae of these two species in the larval form. A taxonomy specialist is required to separate the larvae to species. The moths, however, are relatively easy to separate and this is important. Both moth species will come to the cabbage looper pheromone.
Cabbage Looper: The larvae of loopers can be distinguished from other caterpillars by their distinctive looping movement, in which they arch the middle of their bodies so their front legs meet their back legs. Larvae are pale green and have a narrow white stripe along each side and several down the back. They grow up to about 25 mm long. Larvae have three pairs of legs on the thorax and three pairs of prolegs on the abdomen (one pair on segments five and six and one pair on the terminal segment). Adults are brown moths with a distinctive "dog leg" on their forewings. The dog leg is thicker and sometimes breaks up into two pieces on the cabbage looer compared to the alfalfa looper. The moths of the cabbage looper are slightly smaller and slightly duller in color than the alfalfa looper.
Alfalfa Looper: Adults have silvery-gray forewings marked with an ivory colored "dog leg" mark similar to that found on the forewings of cabbage looper. The dog leg on the alfalfa looper is thinner and brighter compared to the cabbage looper. Alfalfa loopers are larger and brighter in color than cabbage loopers with a wingspan of 30 to 40 mm. Alfalfa larvae closely resemble the cabbage looper in color, but usually have a dark top stripe edged with white lines and two obscure white top-lateral lines. Again, it is difficult to separate the larvae of these two species in the field.
Lifecycle
Cabbage Looper: Female moths can lay 200-350 eggs, usually over a 10-12 day period. Eggs are white, round and about the size of a pinhead. They usually lay eggs singly near the outer fringes of lower leaves of crops. Larvae hatch 3 to 6 days after being laid. They immediately begin feeding in the underside of the leaf producing small holes that do not break through the upper surface of the leaf. The larvae will feed for 2 to 4 weeks. Development of eggs and larvae will be slower during the cooler months of fall. There are three or four generations each year.
Alfalfa Looper: Alfalfa loopers overwinter as pupae either in soil or in trash near the base of host plants. Moths begin emerging in late spring and adults lay eggs singly on weed hosts (mostly wild crucifers). Eggs hatch in 3 to 5 days and larvae feed for about two weeks before pupating in cocoons on the host plant or in trash. The total development time from egg to egg requires about 30 days. Adults emerge in about seven days, mate, and females deposit eggs as before about three days after emerging. Damage is most evident in June and July and again in September and October. There are three or four generations each year.
Crops Affected & Damage
Cabbage and Alfalfa Looper larvae feed on leaves, causing ragged-edge holes in the leaf and on the leaf margins. The major damage caused by larvae and pupae of the cabbage looper is contamination of the heads. In an outbreak year, Alfalfa loopers have sometimes defoliated alfalfa, peas, sugar beets, beans, mint, and spinach.
Scouting & Monitoring
Pheromone traps can be used to monitor the emergence of adult male moths. There are two egg laying flights per year. You will catch both alfalfa and cabbage looper moths in the same trap using the cabbage looper lure. This can lead to misreadings. The cabbage looper is the primary contaminant for harvested broccoli and the primary target of pest control tactics. Scouts need to identify and count both species.
We recommend the use of the large, Texas style wire-mesh pheromone traps placed at the edge of the field. We have not been successful getting useful and consistent reading using the disposable paper wing type pheromone traps.

Texas Style Wire-Mesh pheromene trap (Picture by Pamela Opfer)
Interpreting Flight Data
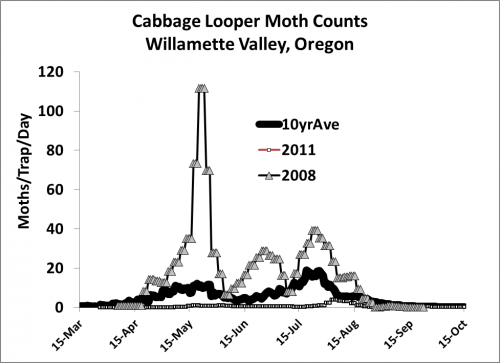
(Figure by Dan McGrath)
The figure above shows high, low, and ten year average moth counts for the cabbage looper in the Willamette Valley. Peak numbers in a normal year reach as high as twenty moths per trap per day. During an outbreak year, counts can exceed one hundred moths per trap per day.
Assessing Risk
There are a number of insects that contaminate broccoli and cauliflower including the cabbage looper, the diamondback moth, the cabbage white butterfly, Bertha armyworm, and the cabbage aphid. In any given planting, the likelihood of all five of these insects being absent at the same time is low. Therefore, we recommend that all broccoli and cauliflower plantings receive a “clean up” insecticide spray seven to ten days prior to harvest. In other words, we do not recommend a zero spray program for broccoli and cauliflower.
When pressure is normal or below normal, uses a one spray or “cleanup” spray program. Additional insecticide applications should be applied prior to the cleanup spray at the button stage, 14 to 21 days prior to harvest. The earlier sprays should be based on field scouting.
Do not make spray decisions based on pheromone trap counts along. Make spray decisions based on a combination of pheromone trap moth counts and field scouting. Pheromone moth trap counts indicate when to intensify field scouting.
Field scouting should begin when the broccoli or cauliflower buttons are one centimeter in diameter, about the diameter of a dime. While crossing the field rows, pull one leaf (mature but not old) from each row. Every ten rows, examine the ten leaves, record the number and species of eggs, larvae, and pupae. Then, continue crossing the field. Collecting another ten leaves, stop, identify, and record. Repeat the procedure until you have examined a minimum of 100 leaves.
If you find three to five large looper worms or more per 100 leaves at the button stage, you may need to spray twice. The first spray should be applied before the broccoli and cauliflower buttons start to elongate, when the buttons are about three centimeters in diameter (about the size of a fifty cent piece). A second spray, the cleanup spray should be applied seven to ten days prior to harvest. We do not recommend a single spray program if the first spray is applied at the button spray. If you apply an insecticide at the button stage, you are committing to a two-spray program. Always apply a cleanup spray seven to ten days prior to harvest.
In a severe outbreak year, growers receive advanced warning via the VegNet Regional Pest Monitoring system. In a severe outbreak year, it may be necessary to apply a third, short residual insecticide in between harvests. Intensive scouting before and after insecticide applications is required during an outbreak year to determine if follow up insecticides are needed.
Control Methods
Biological Control: Looper population are highly regulated by weather, irrigation, disease, and to a limited extent by natural enemies, predators and parasites. It is not uncommon for a pest population to crash the year following a pest outbreak. Biocontrol is important, but it is very difficult to predict. Biocontrols generally follow an outbreak; they rarely regulate outbreaks. They rarely are sufficient to reduce heavy infestations. If treatment is necessary but pressure is low, consider the use of microbial insecticides (Bacillus thuringiensis, Spinosid). They will suppress pest populations while minimizing injury to natural enemies.
Cultural Control: There are no effective cultural controls for this pest.
Chemical Control: Insecticides in general and microbial insecticides in particular are much more effective against small larvae than large larvae. Spray when larvae are small, just following egg laying. Use a combination of pheromone trap moth counts to detect egg laying and field scouting to track the progress of the larval development. Once the insects have formed their pupa inside broccoli or cauliflower heads, it is too late to control crop contamination. Insecticide treatments are ineffective; they do not remove the contaminant. Control insect pests in broccoli and cauliflower before they form their pupae. Please consult the PNW Insect Management Handbook for pesticide recommendations.
References and Citations
"Alfalfa Looper Factsheet." Oregon State University. Accessed December 8, 2011. (http://insects.ippc.orst.edu/pdf/reb34.pdf).
Berry, Ralph E. 1998. "Alfalfa Looper Factsheet." Modified from Insects and Mites of Economic Importance in the Northwest. 2nd Ed. p. 221. (http://mint.ippc.orst.edu/loopfact.pdf).
"Cabbage Looper Factsheet." Oregon State University. Accessed December 8, 2011. (http://insects.ippc.orst.edu/pdf/reb35.pdf).
Hollingsworth, Craig S. (Ed.). 2011. Cabbage and Alfalfa Looper in Mint. Pacific Northwest Insect Management Handbook. Corvallis: Oregon State University.
Natwick, E.T. 2010. "UC IPM Pest Management Guidlines: Cole Crops." University of California Agriculture & Natural Resources. (http://www.ipm.ucdavis.edu/PMG/r108301011.html).
